Insulin Resistance: Unveiling the Culprits
- S A
- Feb 27
- 7 min read
Updated: Mar 3
Part 4
Insulin resistance is a major health concern, often linked to type 2 diabetes and metabolic syndrome. It occurs when your cells become less responsive to insulin, a hormone that helps regulate blood sugar levels. Understanding the root causes of insulin resistance is crucial for developing effective prevention and management strategies.
Where Does Insulin Resistance Come From? Picking up from our part 3, where we looked into the role of Insulin's role in a hot of metabolic conditions, now let us look at the primary culprits behind Insulin Resistance.
Chronic Inflammation
This low-grade, simmering inflammation throughout the body throws a wrench into insulin's ability to function properly. Think of insulin as a key that unlocks cells, allowing them to absorb glucose (sugar) from the bloodstream. Inflammation can damage these cellular "locks" or the insulin "key" itself, hindering glucose uptake.
Elevated insulin levels (hyperinsulinemia) also drive chronic low-grade inflammation by:
Increasing pro-inflammatory cytokines like TNF-α and IL-6, which contribute to endothelial dysfunction.
Promoting oxidative stress, making LDL particles more prone to oxidation—a key step in plaque formation.
Disrupting normal lipid metabolism, leading to higher triglycerides, more remnant lipoproteins, and an unfavourable lipid profile.
Cortisol/Epinephrine
These hormones, often referred to as the "fight-or-flight" hormones, have a significant impact on blood sugar levels. When we experience stress, our body releases cortisol and epinephrine to prepare for a perceived threat. These hormones cause the liver to release glucose into the bloodstream, providing a quick energy source. However, chronically elevated cortisol levels due to constant stress can lead to:
Liver Glycogenolysis: Cortisol and epinephrine act on the liver, stimulating the breakdown of stored glycogen (the storage form of glucose) into glucose. This releases a quick burst of energy into the bloodstream for muscles to use during the stressful situation.
Gluconeogenesis: In some cases, cortisol can also stimulate gluconeogenesis, the process of creating new glucose from non-carbohydrate sources like proteins and fats. This provides an additional source of blood sugar for sustained energy needs during prolonged stress.
Decreased Insulin Sensitivity: Both cortisol and epinephrine can also indirectly increase blood sugar levels by decreasing the body's sensitivity to insulin. Insulin is a hormone produced by the pancreas that helps cells absorb glucose from the bloodstream. When cortisol and epinephrine are elevated, they can interfere with insulin signaling, making it harder for cells to take up glucose. This allows more glucose to remain circulating in the bloodstream, further contributing to hyperglycemia (high blood sugar).
Hyperinsulinemia
This refers to chronically high levels of insulin circulating in the bloodstream. While insulin's job is to lower blood sugar, constantly having elevated insulin can backfire. Here's why:
Receptor Downregulation: Imagine insulin receptors on cell surfaces as tiny mailboxes. When insulin arrives (the mail), it signals the cell to open the mailbox and take up glucose from the bloodstream. However, with constantly high insulin levels, these receptor "mailboxes" become overloaded. The cells eventually downregulate the receptors, meaning there are fewer available to receive insulin's message. This leads to insulin resistance, as cells become less responsive to insulin's signal.
Hyperinsulinemia and Insulin Resistance
Hyperinsulinemia—chronically elevated insulin levels—is not just a symptom of metabolic dysfunction; it is a direct driver of insulin resistance. A striking demonstration of this was seen in a study where lean, healthy individuals (ages 18-25) with normal fasting insulin levels (~8 μU/mL) were given a low-dose insulin infusion, raising their fasting insulin to just 20 μU/mL—still within what many would consider a "normal" range.
Within 48 to 72 hours, these previously insulin-sensitive individuals developed insulin resistance comparable to that of type 2 diabetic patients. This study highlights a crucial, yet often overlooked, fact:
➡ Hyperinsulinemia itself can induce insulin resistance, independent of diet, obesity, or other metabolic factors.
This is why chronically high insulin levels should not be ignored, even in individuals who appear metabolically "normal." It sets off a vicious cycle where the body becomes resistant to insulin, requiring even more insulin to maintain glucose control—pushing the system further into metabolic dysfunction. Over time, this not only contributes to diabetes but also increases inflammation, dyslipidemia, and cardiovascular disease risk.
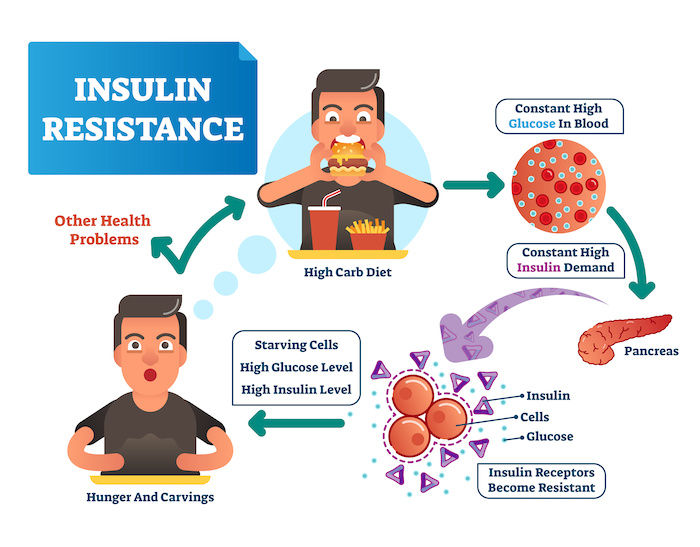
Image Credit: Unified Care
Pathophysiology of Insulin Resistance in the Context of Hyperinsulinemia
Insulin resistance develops when cells in the body—particularly in the liver, muscle, and adipose tissue—fail to respond adequately to insulin. Hyperinsulinemia, or chronically elevated insulin levels, plays a central role in this process by triggering multiple pathological mechanisms that contribute to insulin resistance.
Persistent Insulin Signaling Leads to Receptor Downregulation
Normal insulin function: Insulin binds to the insulin receptor on cell membranes, activating a cascade of intracellular signaling that allows glucose uptake, particularly in muscle and fat cells.
Chronic hyperinsulinemia: When insulin levels remain elevated for extended periods, insulin receptors become desensitized due to continuous stimulation.
Result: The body reduces the number of insulin receptors and decreases their sensitivity, requiring even more insulin to achieve the same effect, thereby exacerbating insulin resistance.
Disruption of Insulin Signaling Pathways
Insulin acts via two primary pathways:
Metabolic Pathway (PI3K-Akt Pathway): Regulates glucose uptake, glycogen synthesis, and lipid metabolism.
Mitogenic Pathway (MAPK Pathway): Influences cell growth and inflammation.
In insulin resistance:
The PI3K-Akt pathway becomes impaired, reducing glucose uptake and leading to elevated blood sugar levels.
The MAPK pathway remains active, promoting inflammation and oxidative stress, which further contributes to insulin resistance.
Lipotoxicity and Ectopic Fat Deposition
Hyperinsulinemia promotes fat storage by stimulating lipogenesis in the liver and adipose tissue.
Over time, excess fatty acids spill over into non-adipose tissues such as the liver, muscle, and pancreas.
Ectopic fat deposition (lipotoxicity) disrupts normal insulin signaling and promotes inflammation, further impairing insulin action.
Inflammatory Cascade and Cytokine Release
Chronically high insulin levels lead to increased production of pro-inflammatory cytokines such as TNF-α, IL-6, and CRP.
These cytokines impair insulin receptor function and promote oxidative stress, worsening insulin resistance.
Adipose tissue, especially visceral fat, becomes inflamed and releases more cytokines, creating a vicious cycle of worsening insulin resistance.
Hepatic Insulin Resistance and Increased Glucose Production
In a healthy state: Insulin suppresses gluconeogenesis (glucose production) in the liver.
In insulin resistance: The liver becomes less responsive to insulin, leading to excessive glucose release into the bloodstream, worsening hyperglycemia.
Despite high insulin levels, the liver behaves as if the body is in a fasting state, continually producing glucose.
Muscle Insulin Resistance and Reduced Glucose Disposal
Skeletal muscle is the largest site for glucose uptake in the body.
Insulin resistance in muscle reduces GLUT4 translocation, meaning glucose cannot efficiently enter muscle cells.
This leads to persistently high blood sugar levels, further stimulating insulin secretion and perpetuating hyperinsulinemia.
The Vicious Cycle of Hyperinsulinemia and Insulin Resistance
Chronic hyperinsulinemia is both a driver and consequence of insulin resistance.
As cells become resistant to insulin, the pancreas compensates by secreting more insulin to maintain normal blood sugar levels.
This further desensitizes insulin receptors, worsening the resistance.
Over time, pancreatic β-cells become overworked and may begin to fail, leading to β-cell dysfunction and type 2 diabetes.
Mechanism | Process | Effect on Insulin Resistance |
Receptor Downregulation | Chronic high insulin levels cause reduced insulin receptor expression and sensitivity. | Cells require more insulin to respond, worsening resistance. |
Disrupted Insulin Signaling | The PI3K-Akt pathway (glucose uptake) is impaired, while the MAPK pathway (inflammation) remains active. | Reduced glucose uptake, increased inflammation, and oxidative stress. |
Lipotoxicity & Ectopic Fat | Excess fatty acids accumulate in the liver, muscle, and pancreas instead of adipose tissue. | Fat deposits impair insulin signaling and increase inflammation. |
Inflammatory Cytokine Release | Chronic hyperinsulinemia stimulates pro-inflammatory cytokines (TNF-α, IL-6, CRP). | Cytokines interfere with insulin receptors, worsening insulin resistance. |
Hepatic Insulin Resistance | The liver fails to suppress glucose production despite high insulin levels. | Increased fasting glucose levels, contributing to hyperglycemia. |
Muscle Insulin Resistance | Impaired GLUT4 translocation prevents glucose uptake into muscle cells. | Blood sugar remains elevated, requiring more insulin secretion. |
Vicious Cycle of Hyperinsulinemia | Pancreas compensates by producing more insulin, further desensitizing insulin receptors. | Worsening insulin resistance, leading to β-cell dysfunction and potential type 2 diabetes. |
This structured table simplifies the complex mechanisms behind hyperinsulinemia-induced insulin resistance while highlighting its cascading effects.
Key Takeaways
Hyperinsulinemia is not just a response to insulin resistance—it actively drives it by impairing insulin receptor function, disrupting signalling pathways, promoting inflammation, and causing ectopic fat accumulation.
Chronic insulin elevation promotes a vicious cycle where higher insulin levels are required to compensate for resistance, further worsening metabolic dysfunction.
Targeting hyperinsulinemia through dietary and lifestyle interventions (e.g., low-carbohydrate diets, exercise, fasting) can help break this cycle and improve insulin sensitivity.
This sets the stage for metabolic diseases such as type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD). Therefore, addressing hyperinsulinemia early is crucial in preventing long-term metabolic dysfunction.
Conclusion: The Vicious Cycle of Hyperinsulinemia and Insulin Resistance
Hyperinsulinemia is both a driver and a consequence of insulin resistance, creating a self-perpetuating cycle that disrupts metabolic balance. As insulin levels remain chronically elevated, cells become less responsive, forcing the pancreas to produce even more insulin to compensate. This overproduction not only accelerates insulin resistance but also fuels systemic inflammation, as high insulin levels trigger pro-inflammatory cytokines and oxidative stress.
At the same time, chronic stress—whether physiological (poor diet, obesity, sedentary lifestyle) or psychological (cortisol dysregulation, sleep deprivation)—exacerbates insulin resistance by further impairing insulin signaling and increasing inflammatory markers. The combination of insulin resistance, chronic inflammation, and stress creates a metabolic environment that significantly raises the risk for conditions like type 2 diabetes, cardiovascular disease, and even neurodegenerative disorders.
Breaking this cycle requires a multi-faceted approach: lowering insulin levels through diet, exercise, and stress management, reducing inflammation with proper nutrition and lifestyle interventions, and improving insulin sensitivity by addressing the root causes of metabolic dysfunction. Understanding hyperinsulinemia as both a symptom and a driver of these interconnected processes is crucial in reversing its damaging effects.
*Disclaimer:
The information provided in this blog is for educational and informational purposes only and should not be construed as medical advice. While every effort is made to ensure accuracy, the content is not intended to replace professional medical consultation, diagnosis, or treatment. Always seek the guidance of a qualified healthcare provider with any questions regarding your health, medical conditions, or treatment options.
The author is not responsible for any health consequences that may result from following the information provided. Any lifestyle, dietary, or medical decisions should be made in consultation with a licensed medical professional.
If you have a medical emergency, please contact a healthcare provider or call emergency services immediately.


Comments